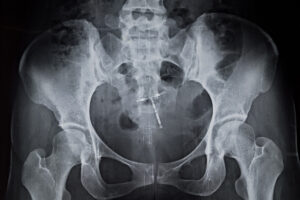
Understanding Settlement Offers: Should You Accept or Fight for More?
When faced with a personal injury claim, an insurance company may present a settlement offer. This proposal may seem appealing, but carefully weighing all options remains crucial. Accepting the offer